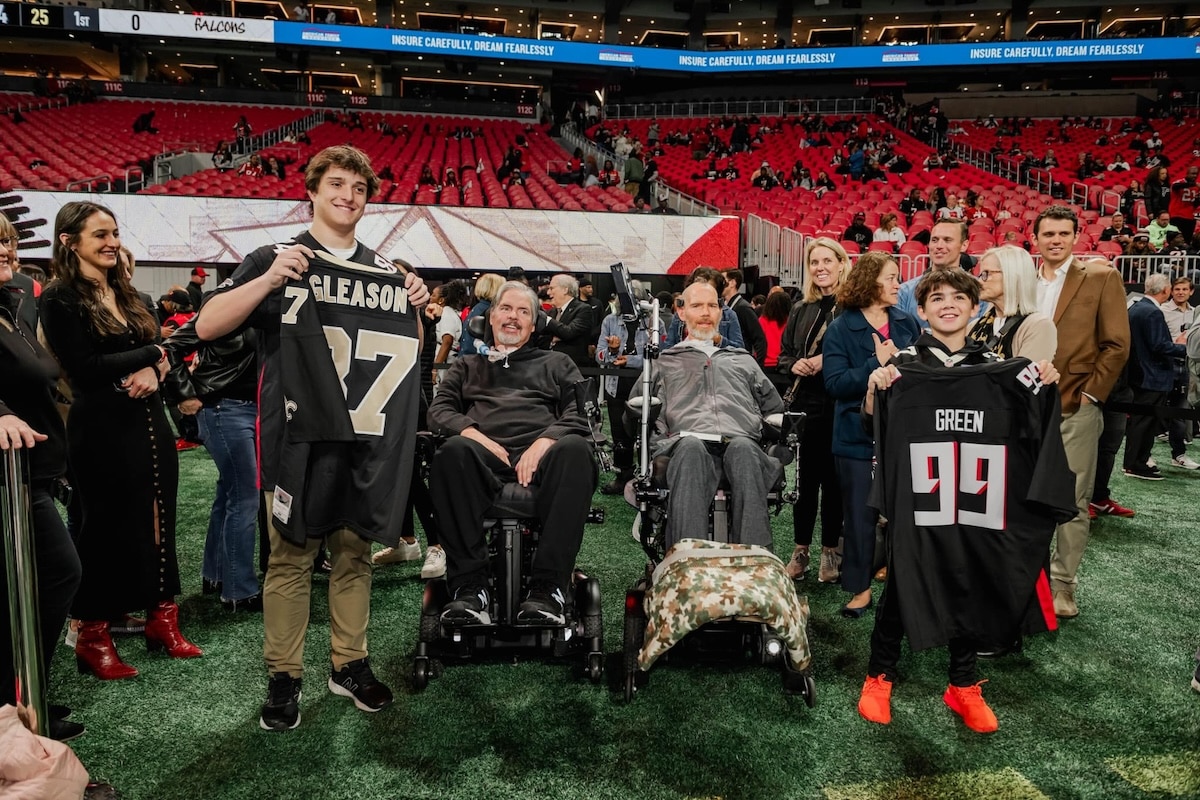NEWS
April 9, 2024
The Center’s annual impact report is now available for viewing and download. Click here to read about our continued progress.
February 15, 2024
The Robert Packard Center for ALS Research at Johns Hopkins announces the appointment of Jim Halpert to its Board of Governors. “Jim brings talent, expertise, and energy to our fight to end ALS. As an ALS patient, he brings a personal and unique perspective to the Board,” noted Jeffrey Rothstein, MD, PhD, Packard Center founder […]
February 5, 2024
Ruth WendlandtReprinted with permission: https://giving.jhu.edu/story/packard-center-als-calendar-girls/ The coast of Lake Michigan in Sheboygan, Wisconsin, was the setting for an enduring friendship among 13 women. Together they celebrated many milestones, including their 1972 graduation from North High, college commencements, and weddings. But a health diagnosis reunited the classmates back to their hometown to support their friend Wendy […]
February 3, 2024
Reprinted from www.livelikelou.org As part of our commitment to invest in people, places, and discoveries that will lead to tomorrow’s trials, treatments, and cures for ALS, we are proud to announce that Evangelos Kiskinis, PhD, from Northwestern University will serve as the Live Like Lou Foundation’s Scientific Director. The Scientific Director will drive Live Like […]
December 6, 2023
Packard Center investigator, Dr. Alyssa Coyne, received 15th Annual Paulo Gontijo Award from the Instituto Paulo Gontijo. The prize is awarded to promising young ALS researcher. It was presented to Dr. Coyne at the opening of the 34th International Symposium on ALS/MND in Basel, Switerland. Dr. Coyne is an Assistant Professor in the Department of […]
November 28, 2023
Tim Green, Atlanta Falcons Legend, and Steve Gleason, New Orleans Saints Legend, have something in common well beyond football and have leveraged their platforms for a cause greater than life on the field. Watch their story: https://www.youtube.com/watch?v=gzwS4LN6f5s
November 28, 2023
Gifts made between Giving Tuesday and December 31 will be matched up to $100,000. On this national day of giving, you can support ALS research and accelerate breakthroughs by making your gift today. Your generosity will enrich groundbreaking research and impact the speed at which discoveries can be made by our Packard Center – funded […]
November 28, 2023
ALS Advocate Brian Wallach recently appeared on CBS Sunday Morning to discuss his battle against ALS. Brian Wallach has beaten the odds after being diagnosed with ALS (amyotrophic lateral sclerosis, or Lou Gehrig’s disease). Given six months to live, remarkably he has survived six years. During that time, he and his wife Sandra have lobbied […]
November 10, 2023
Packard Center founder and director, Dr. Jeffrey Rothstein, was a panelist at the recent Milken Institute Future of Health Summit in Washington, DC. He appeared on the panel entitled: Future Pathways to Enable Open Science and Increase Research Transparency and Collaboration where he spoke with experts from the Aspen Institute, Good Science Project, The Latino […]
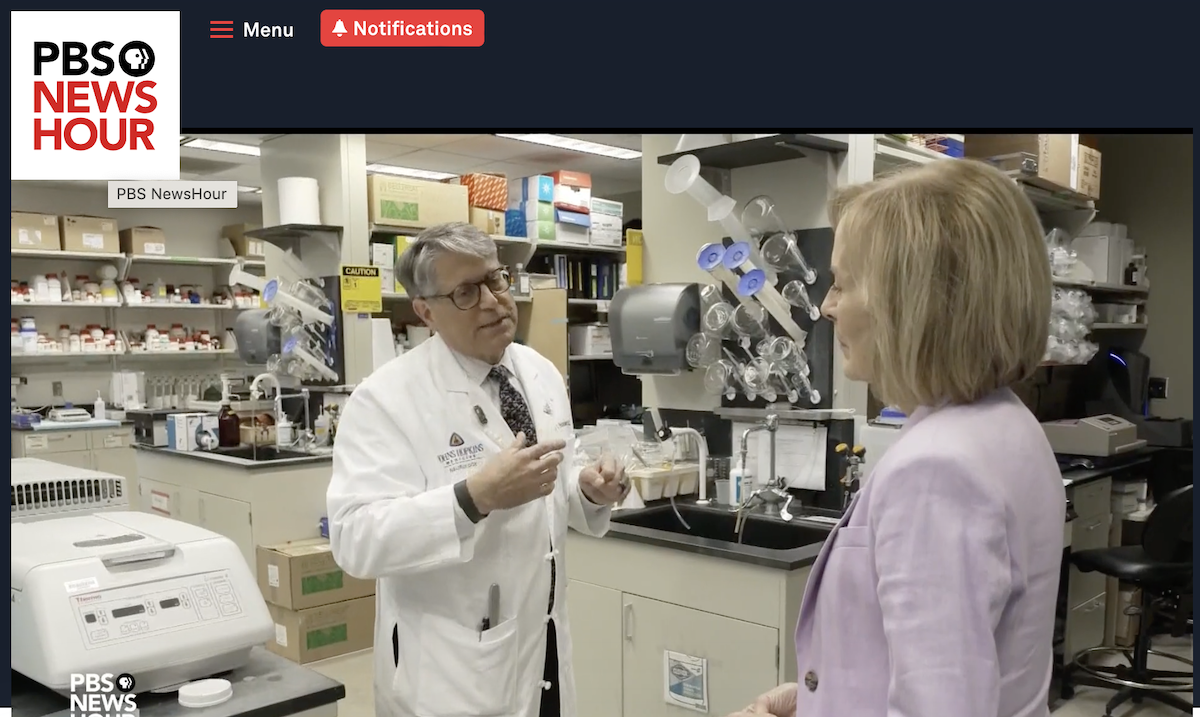
November 2, 2023
Packard Center founder and director, Dr. Jeffrey Rothstein, recently appeared with Judy Woodruff on the PBS News Hour discussing advances in ALS Research. The piece aired on Wednesday, November 1, 2023. Watch here: https://www.pbs.org/newshour/show/former-nyc-deputy-mayor-raises-millions-for-als-research-while-facing-his-own-mortality